Deep brain stimulation (DBS) has been approved for the treatment of movement disorders since the 1990s. Numerous studies have proven this surgery’s superiority to medical therapy alone. The time to consider DBS surgery is when the quality of life is no longer acceptable on optimal medical therapy as administered by a movement disorder specialist. DBS surgery is very safe and recent advancements in device technology have improved patient outcomes as well.
Parkinson Disease
DBS surgery offers significant symptomatic relief in patients with moderate disability from Parkinson's disease. DBS therapy improves the motor symptoms of Parkinson’s including tremor (shaking), rigidity (stiffness), and bradykinesia (slowness). In short, DBS improves the symptoms that medication (mainly levodopa) improves, but DBS offers smoother therapy throughout the day that reduces fluctuations between “off” and “on” periods. DBS is likely to improve tremor even if it does not respond to medication. Also, it’s a common misconception that DBS is a treatment for Parkinson’s tremor only. Even patients without tremor see improvement with DBS therapy. DBS can also reduce involuntary movements called dyskinesia, and patients may be able to reduce their medication doses after surgery, although surgery will not replace medication entirely.
Download the DBS for Parkinson's Disease handout (pdf)
Essential Tremor
DBS is a highly effective therapy for patients with essential tremor, often resulting in tremor reduction of 80% or more. DBS works well to reduce tremor of the hands, but it also improves tremor in other body parts such as the head, voice, and legs. Patients with essential (familial) tremor that have not had an adequate response to at least 1 or 2 medications and whose tremor impairs their quality of life are likely to be good candidates for DBS. Patients with a tremor secondary to stroke, traumatic brain injury or multiple sclerosis are less likely to benefit from DBS, but may still be surgical candidates if expectations are realistic.
Download the DBS for Essential Tremor handout (pdf)
Dystonia
DBS decreases the abnormal movements and postures of dystonia, especially in generalized, primary, and inherited forms of the disorder. The degree of benefit appears to vary with both the type of dystonia and the duration of the symptoms. DBS may help patients with other forms of dystonia if medical therapy fails, but the response may be less.
Meige Syndrome
Also known as oral-facial dystonia, this syndrome is a combination of two forms of dystonia: blepharospasm and oromandibular dystonia. Movement disorder doctors work closely the Cranial Nerve Disorders Program, to identify patients with Meige Syndrome who may benefit from DBS.
Who Should get Deep Brain Stimulation (DBS)?
This is a common question with a surprisingly simple answer: Anyone who would get significant benefit from the treatment and can undergo the operation with minimal risk. It is not necessary to suffer for years after diagnosis, trying every known combination of medicine, before DBS can be considered. DBS is a surgical option that is known to improve quality of life for patients with movement disorders, so when one’s quality of life is dramatically affected by the disease or by medication side effects, it’s time to consider DBS.
What is DBS?
DBS surgery involves placing a thin electrode (about the diameter of a piece of spaghetti) into one of several possible brain targets on one or both sides of the brain. The electrode is connected to a battery, which is implanted under the skin in the chest below the collarbone. All parts of the stimulator system are internal; there are no wires coming out through the skin. A programming computer is used during routine office visits to adjust the settings for optimal symptom control. Unlike lesioning procedures such as gamma knife or focused ultrasound ablation, DBS does not permanently destroy brain tissue. Instead, it reversibly alters the function of the brain tissue in the region of the stimulating electrode. It is important to note that DBS therapy may demand considerable time and patience before its effects are optimized.
How Does DBS Work?
DBS is not a cure for movement disorders, but it can successfully treat symptoms by disrupting the abnormal patterns of brain activity that become prominent in these diseases. DBS is often described as a brain “pacemaker” because constant pulses of electrical charge are delivered at settings that are thought to restore normal brain rhythms, allowing the restoration of more normal movements. The exact mechanisms of this neuromodulation are still unknown.
How is the Surgery Performed?
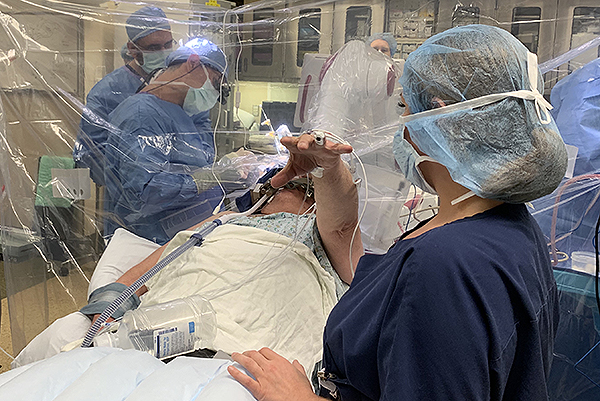
UPMC was one of the first centers to use ROSA robotic assistance for the placement of DBS electrodes. ROSA is similar to a GPS device for the brain. It provides the surgeon with a roadmap to reach the intended brain targets. The patient is sedated for the beginning of the surgery while we make a small opening in the skin and bone at the surgical site(s). The patient will not feel or remember this part of the surgery, but once these steps are complete, he is awoken for the remainder of the surgery.
Brain Mapping
We use neurophysiology recordings from very thin electrodes inserted into the brain to map activity in the intended target and confirm the best spot for the DBS electrode. It is important for the patient to be awake during this part of the surgery so we can obtain the best recordings possible, which will aid in the most accurate placement of the DBS electrode. The brain mapping is not painful and the surgical team will be available to provide reassurance and feedback the entire time.
Intra-Operative Stimulation Testing
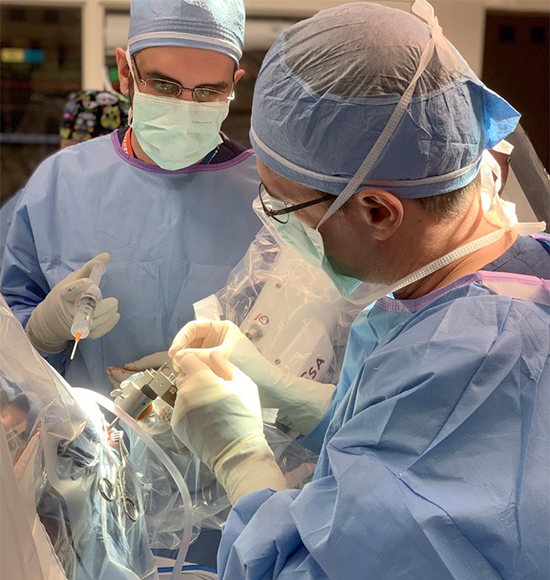
When the best site is identified from the brain mapping, the DBS electrode is inserted and tested. We monitor the patient for improvement in his symptoms, for example tremor, and also ask him to report any new sensations he experiences. Again, this part of the procedure is not painful, but provides valuable feedback to the surgical team.
Once the DBS electrodes are placed, stage 1 of surgery is complete. The patient is typically observed in the hospital for 1 night before going home.
Stage 2
The patient will return an average of 2 weeks later for a second procedure to place the battery and connect it to the brain electrodes. This surgery is very short and performed as an outpatient procedure
We understand that the thought of being awake for part of a brain surgery can be overwhelming and scary. We advise you to discuss your concerns with your surgical team. Accommodations can be considered for patients who are unable or unwilling to participate in an awake surgery.
Types of DBS Systems
At UPMC, we are excited to offer our patients a choice of which DBS system is best-suited for them. There are now 3 different DBS systems available, and our team has experience with all of them. Ultimately, the decision is yours, but our team will help guide you in making this important decision. Please keep in mind that all 3 systems are more alike than different, and all are expected to improve your symptoms. However, each system has its own advantage that sets it apart from the others, and one might fit your lifestyle better than another.
Medtronic Activa™: With their first product approved in 1997, Medtronic is the longest-running manufacturer of DBS equipment. They offer both a non-rechargeable battery and a rechargeable battery that lasts 15 years. Patients are given a patient programmer which they can use to adjust their DBS settings within a pre-set range. This DBS system is MRI compatible.
St. Jude Medical Infinity™(Abbott): In January 2017, UPMC became the first center in Pennsylvania to offer DBS patients directional lead technology. This DBS system has segmented electrodes that allow precise steering of current towards desired structural area to maximize therapeutic benefit and reduce potential side effects. Patients are given iPods which they can use to adjust their DBS settings within a pre-set range. The battery is not rechargeable. This DBS system is MRI compatible.
Boston Scientific Vercise Gevia™: This DBS system has segmented electrodes that allow precise steering of current towards desired structural area to maximize therapeutic benefit and reduce potential side effects. Multiple Independent Current Control (MICC) provides a dedicated power source for each electrode to precisely control stimulation and refine the size and shape of the electrical field. Patients are given a patient programmer which they can use to adjust their DBS settings within a pre-set range. They offer a rechargeable battery that will last at least 15 years. This battery is the smallest DBS battery available. This DBS system is MRI compatible.
