The Laboratory of Brain Tumor Evolution & Therapy, under the direction of Baoli Hu, PhD, is interested in the genetic and epigenetic events contributing to the evolution of brain tumors. The long-term goal of the lab is to achieve a better understanding of brain tumor biology and to develop more effective diagnoses and therapeutic strategies for the treatment of brain cancer.
Cancer is increasingly being viewed as an ecosystem where the cancer cells dynamically evolve and spatiotemporally communicate with surrounding cells and environmental factors. Deciphering this evolutionary complexity allows us to better understand brain tumor initiation, progression, recurrence, and drug resistance. The Brain Tumor Evolution & Therapy Lab is focusing on glioma which is the most common malignant brain tumor in adults, and the most common type of embryonal tumor arising in the central nervous system in childhood. Specific projects are as follows:
1. Modeling the evolution and complexity of brain tumors. Intratumor genetic heterogeneity and phenotypic diversity are the hallmarks of glioma and medulloblastoma, which predict the risk of tumor development, progression, and response to treatment. To delineate the crosstalk mechanisms of these factors, Dr. Hu’s lab is interested in generating various sophisticated models, which can faithfully recapitulate the molecular diversity, cellular heterogeneity, and microenvironmental complexity seen in patient tumors. These models include genetically engineered mouse brain tumor models, syngeneic and humanized mouse brain tumor models, ex vivo brain tumor models (e.g., brain slices), human-in-mouse model systems based on malignant transformation of human neural/cerebellar stem cells driven by subtype-specific genetic/epigenetic alterations, and so on. These models will deepen our understanding of tumor evolutionary dynamics at the molecular and cellular levels. The key regulators in this process are validated as diagnostic biomarkers and therapeutic targets for clinical application.
2. Interrogating consequences of neural stem cell/tumor cell plasticity within the brain tumor microenvironment. Dr. Hu’s lab previously found that glioblastoma stem cells (GSCs) differentiate into endothelial-like cells (GdECs), which recruit host endothelial cells (ECs) to form an invasive niche, resulting in tumor invasiveness and recurrence. They are continuing their efforts to gain a better understanding of the molecular mechanisms of these cancer stem cells, and how they communicate with their surrounding cells (e.g., endothelial cells, microglia/macrophages, astrocytes, etc.), which allows us to develop novel and more effective therapies by targeting critical components of the tumor microenvironment.
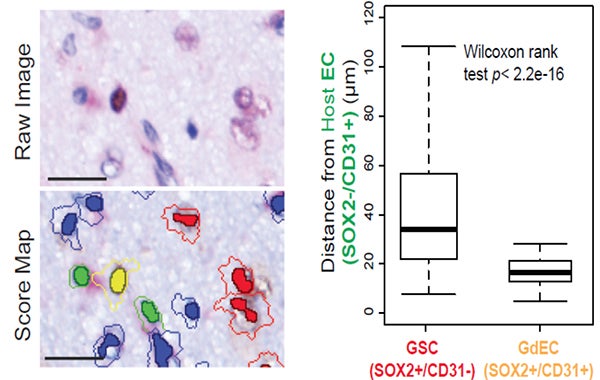
The invasive niche is comprised of GSCs, GdECs, ECs, and other types of cells in GBM patient tumor. Representative images with IHC double-staining and cell segmentation obtained from Caliper InForm analysis software show the close proximity of GdEC (yellow) and host ECs (green) compared with GSCs (red). Cell-cell distance was assessed by an automated quantitative pathology imaging system. Scale bar, 20 mm. (Hu, et al., Cell 167(5),1281-1295, 2016).
3. Targeting glioblastoma immunosuppression in brain tumor immunotherapy. Glioblastoma is highly immunosuppressive and resistant to immunotherapy because glioma cells escape from effective antitumor immunity through programing the tumor microenvironment (TME). Dr. Hu’s lab has recently found that cancer cell-intrinsic signaling reprograms tumor-associated macrophages (TAMs) to mediate tumor suppression by novel protein binding complexes CHI3L1-Gal3-Gal3BP. Their interest is focused on understanding how these protein complexes regulate TAM recruitment, polarization, cytokine production, tumor-infiltrating lymphocyte inactivation, and how to target these protein complexes by developing new drugs in brain tumor immunotherapy.
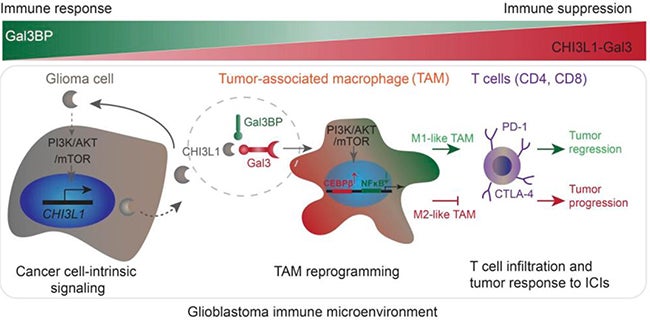
The schematic drawing indicates that glioma cell-intrinsic CHI3L1 binding with Gal3 forms a protein-binding complex modulating the TAM-mediated immune microenvironment for tumor progression, which is negatively regulated by Gal3BP. (Chen et al., J Clin Invest, 2021 Aug 16;131)
4. Illuminating mechanisms governing cancer cell invasion and dissemination in the brain. The major challenge in the clinical management of glioblastoma is that cancer cells extensively infiltrate into the surrounding tissue, leading to nearly universal recurrence. Group 3 medulloblastoma is characterized by frequent metastasis at diagnosis and the worst prognosis among all the subgroups. Dr. Hu’s lab aim is to elucidate molecular mechanisms of de novo invasion and treatment-induced invasion (e.g. TMZ, bevacizumab, etc.), which enables us to identify “drivers” mediating cancer cell invasion and dissemination and to aid in the development of new therapies.
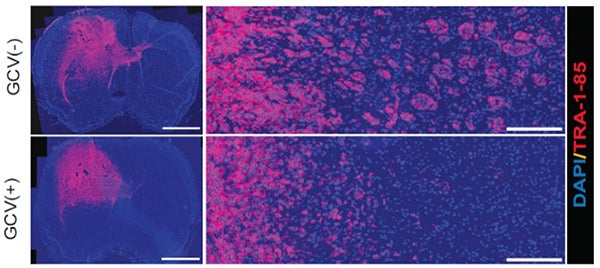
Suppression of tumor invasiveness by depleting WNT5A-mediated GdECs using HSVTK/ganciclovir (GCV) cell ablation system. Representative images for tumor appearance (left, scale bar, 2,000 μm) and peritumoral satellite lesions (right, scale bar, 200 μm) in PDX mouse model. Tumor cells are labeled by staining human specific antigen TRA-1-85 (red). (Hu, et al., Cell 167(5),1281-1295, 2016).
