To aid surgeons in performing more complete and safer resection of brain tumors, new intraoperative technologies have been developed to improve visualization of tumor tissue. Fluorescence-guided surgery (FGS), one of these new technologies, relies on the use of a fluorophore and specific light wavelength to better delineate tumor tissue from surrounding brain tissue. 5-aminolevulinic acid (5-ALA), the first fluorophore developed specifically for brain tumors, accumulates within tumor cells, improving visualization of tumors both at the core and invasive tumor margin. In fact, 5-ALA presence of violet-red fluorescence is highly diagnostic of high-grade glioma—most commonly glioblastoma (GBM)—tumor tissue.
Patients are administered the 5-ALA oral drug, known as Gleolan, hours before their surgery so that during surgery the neurosurgeon can see the tumor tissue light up, allowing for more precise and complete resection. Studies performed have shown that tumors can be resected more completely with FGS in comparison to conventional surgery without the use of 5-ALA and FGS.

At UPMC, our neurosurgeons consistently utilize 5-ALA and FGS for removal of brain tumors. Our neurosurgeons have the latest technology to visualize 5-ALA fluorescing brain tumor tissue that includes a robotic-assisted exoscope. Costas Hadjipanayis, MD, PhD, was the first in the United States to utilize the agent Gleolan for removal of a glioblastoma tumor in 2011. His team has completed a number of clinical studies in the U.S. with 5-ALA confirming safe and effective removal of malignant gliomas. Dr. Hadjipanayis and his team also led the U.S. effort for FDA approval of 5-ALA (Gleolan) for use during glioma surgery in 2017.
In addition to utilization of 5-ALA for visualization of brain tumor tissue, a new formulation of 5-ALA, termed Pentalafen, is new being studied for the first time in the United States by Jan Drappatz, MD, division chief of neuro-oncology at the UPMC Hillman Cancer Center, and Dr. Hadjipanayis, for intraoperative photodynamic therapy (PDT) of glioblastoma (GBM). This form of intraoperative therapy is investigative and is part of a clinical trial unique to UPMC and the Hillman Cancer Center.
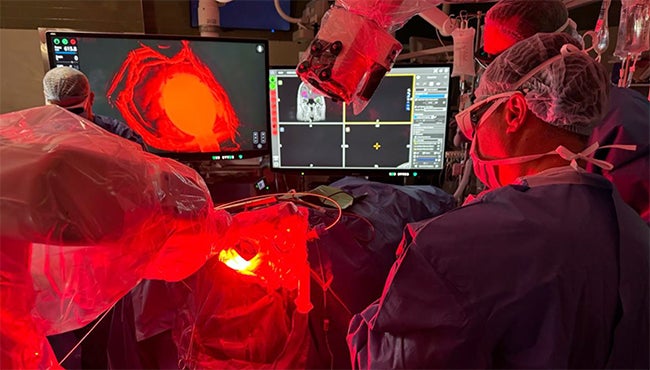
Patients with a suspected GBM tumor are administered the oral agent Pentalafen hours before surgery and then undergo standard 5-ALA FGS. After tumor removal during surgery, a laser light is applied to the resection cavity to potentially target cancer cells at the periphery of the tumor cavity since invisible cells are present. A prior study conducted in Europe has confirmed the intraoperative PDT procedure is safe and effective with newly diagnosed GBM patients.
For any questions related to the ongoing clinical trial please contact us at 412-647-0953 or 412-692-4724.
