Pittsburgh, April 24, 2024 -- Joseph Maroon, MD, noted health and wellness expert and Heindl Scholar in Neuroscience at the University of Pittsburgh Department of Neurological Surgery, presented a talk on SYNC-T—a novel, highly promising, minimally invasive immunotherapeutic approach to treat metastatic solid tumors—while serving as visiting professor at the Norton Neuroscience Institute in Louisville, Ky., April 17. Dr. Maroon is a scientific advisor and an initial founding member of Syncromune, the company pioneering this research.
SYNC-T is an investigational therapy that combines a device-induced vaccination at the tumor site with intratumorally infusion of a multitarget biologic drug. Initial Phase 1 trial results—reported in an invited presentation at the recent American Association for Cancer Research (AACR) Annual Meeting in San Diego—have been very encouraging and have shown numerous positive clinical responses in patients with metastatic castrate-resistant prostate cancer.
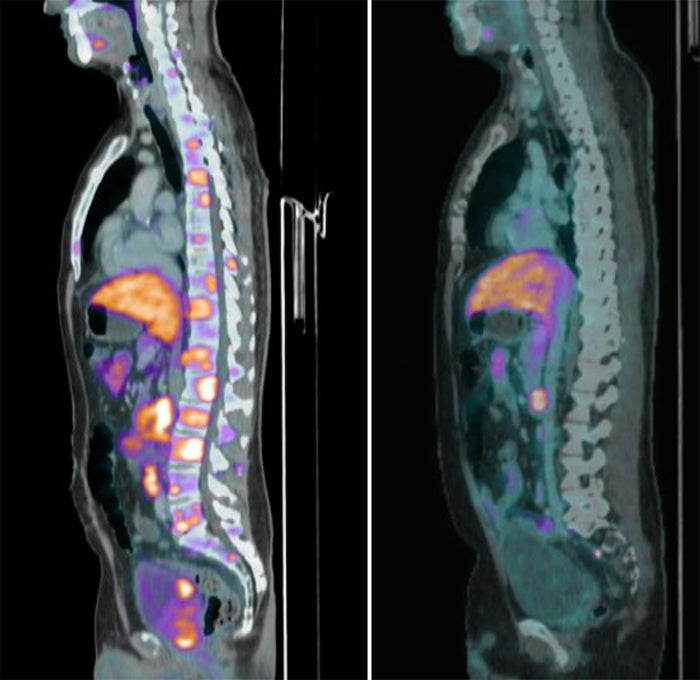
One patient—detailed in the trial—presented with over 50 metastatic bone lesions throughout his spine and pelvis results [see images below]. After seven treatment cycles of SYNC-T SV-102, the subject had complete resolution of all the bone metastases, achieving a complete response.
Of the 13 evaluable patients presented in the trial, 11 experienced an objective response, with five complete responses and six partial responses.
“It is one of the most dramatic results I have seen in my many years of medicine and neurosurgery,” commented Dr. Maroon.
[Watch three-minute animation explaining how SYNC-T Therapy works.]
Jason Williams, MD, an interventional oncologist who pioneered the development of this new therapy is also having some success using the new treatment approach with other solid tumors including intractable metastatic pancreatic, breast and lung cancers as part of other investigational trials.
Syncromune plans to file an Investigational New Drug application with the Food and Drug Administration in the next few months. Upon FDA approval, the company will begin a Phase 1b/2 trial of SYNC-T SV-102 for metastatic castrate-resistant prostate cancer in the second half of 2024.
